ICARIA-MM: SARCLISA + POMALIDOMIDE AND DEXAMETHASONE (Pd)
ICARIA-MM Trial Results: SARCLISA + Pd vs Pd Alone
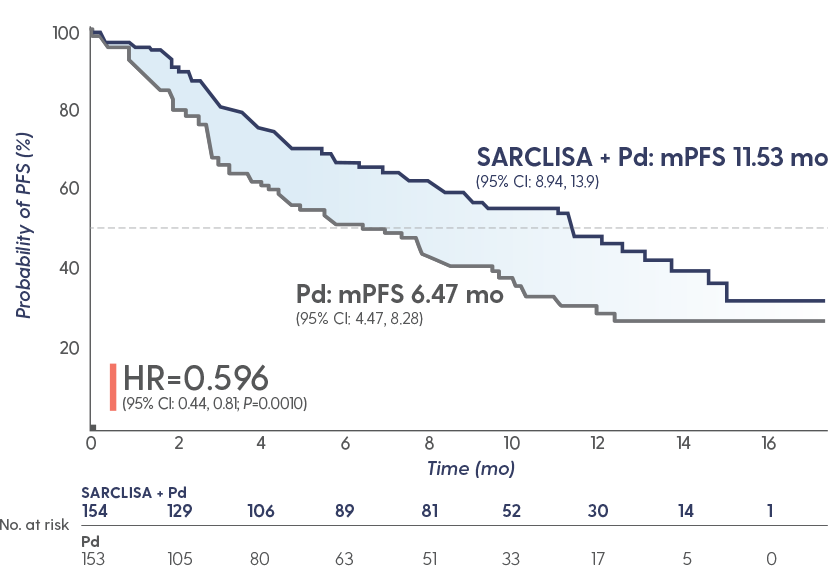
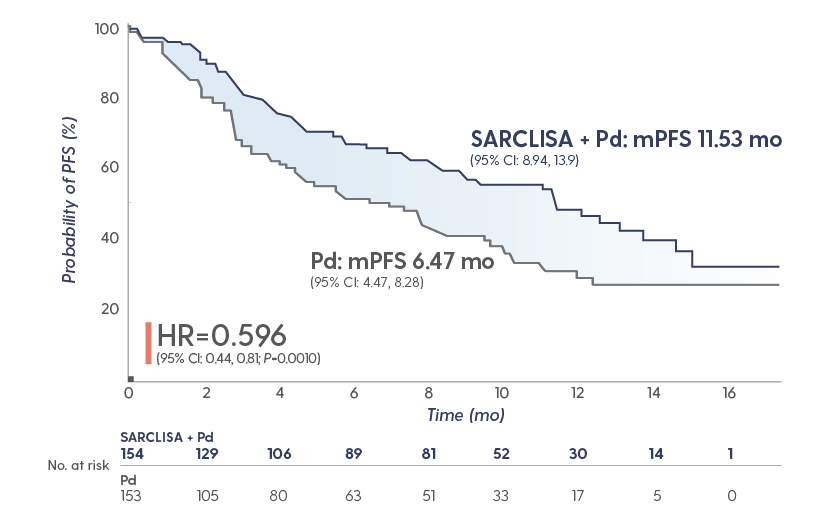
SARCLISA + Pd extended median PFS to ~1 year
Superior PFS with SARCLISA + Pd vs Pd alone1
At a median follow-up time of 52.4 months, final median OS was 24.6 months in the SARCLISA + Pd arm and 17.7 months in the Pd arm (HR=0.776 [95% CI: 0.594, 1.015]).
The median duration of treatment was
41 weeks with SARCLISA + Pd vs 24
weeks with Pd alone
GREATER THAN 40% REDUCTION IN THE RISK OF PROGRESSION OR DEATH
in patients receiving SARCLISA + Pd
mPFS=median progression-free survival; OS=overall survival.
National Comprehensive Cancer Network® (NCCN®) recommends isatuximab-irfc (sarclisa) in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma as a Category 1 Preferred option in combination with carfilzomib and dexamethasone or with pomalidomide and dexamethasone2:
- ✓ For early relapses (1-3 prior therapies)*
- ✓ Option for patients refractory to either lenalidomide or bortezomib
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Recommendation for isatuximab-irfc (SARCLISA) in combination with carfilzomib and dexamethasone based on results of interim analysis.
*After 2 prior therapies including lenalidomide and a proteasome inhibitor for isatuximab-irfc in combination with pomalidomide and dexamethasone.
NCCN=National Comprehensive Cancer Network® (NCCN®).
SARCLISA + Pd showed a significant
increase in ORR1

ORR: SARCLISA + Pd (95% CI: 0.52, 0.68), Pd (95% CI: 0.28, 0.43). 95% CI estimated using the Clopper-Pearson method.
CR=complete response; PR=partial response; sCR=stringent complete response; VGPR=very good partial response.
Median time to first response was 35 days with SARCLISA
+ Pd vs 58 days with Pd alone among responders1