SARCLISA + BORTEZOMIB,
LENALIDOMIDE, AND
DEXAMETHASONE (VRd)
IMROZ: SARCLISA + VRd vs VRd Alone in Patients With NDMM Not Eligible for Transplant
SARCLISA + VRd: A benchmark for mPFS vs VRd alone1-4
Higher 5-year PFS rate with SARCLISA + VRd vs VRd alone: 63% of patients remained alive and progression free at a median follow-up of 60 months1,2
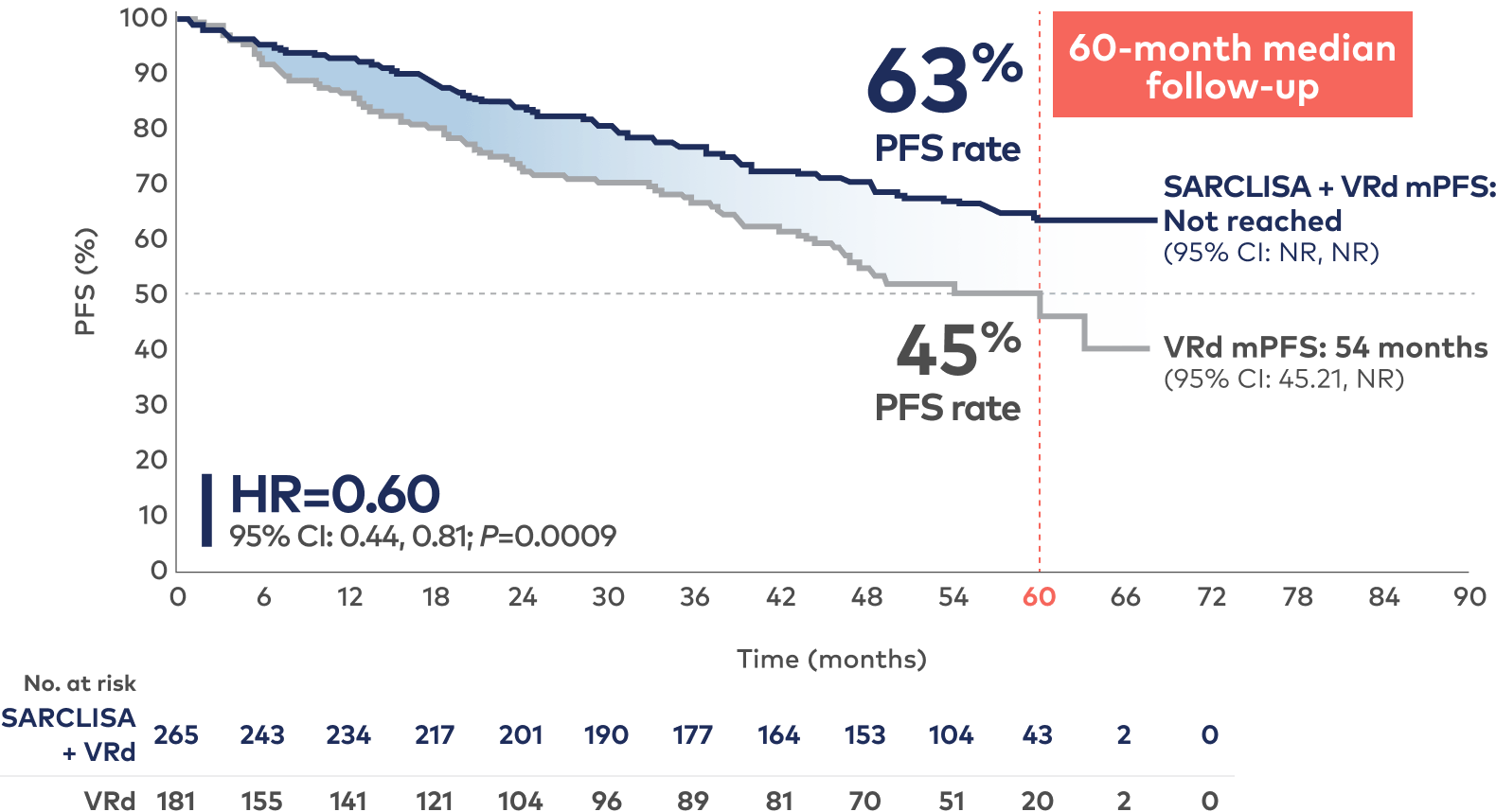
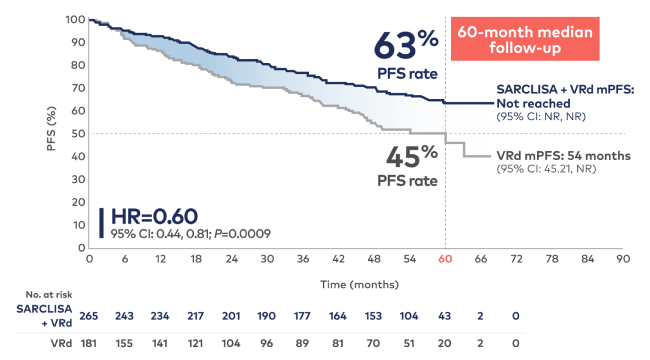
PFS results were assessed by an IRC based on central laboratory data for M-protein and central radiologic imaging review using the IMWG criteria.1
SARCLISA + VRd mPFS: Not reached at 60 months1
What is the mPFS of your preferred non-transplant NDMM regimen?


IMWG=International Myeloma Working Group; IRC=independent response committee; M-protein=monoclonal protein; mPFS=median progression-free survival; NDMM=newly diagnosed multiple myeloma; NR=not reached; PFS=progression-free survival.
NCCN
CATEGORY 1
PREFERRED
National Comprehensive Cancer Network® (NCCN®) recommends isatuximab-irfc (SARCLISA), bortezomib, lenalidomide, and dexamethasone as a NCCN Category 1, Preferred Regimen for the treatment of patients with newly diagnosed multiple myeloma who are non-transplant candidates (<80 years old who are not frail)5
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
NCCN=National Comprehensive Cancer Network® (NCCN®).
Deep and durable responses to extend frontline remissions for your patients6
Superior rate of ≥CR with SARCLISA + VRd vs VRd alone1
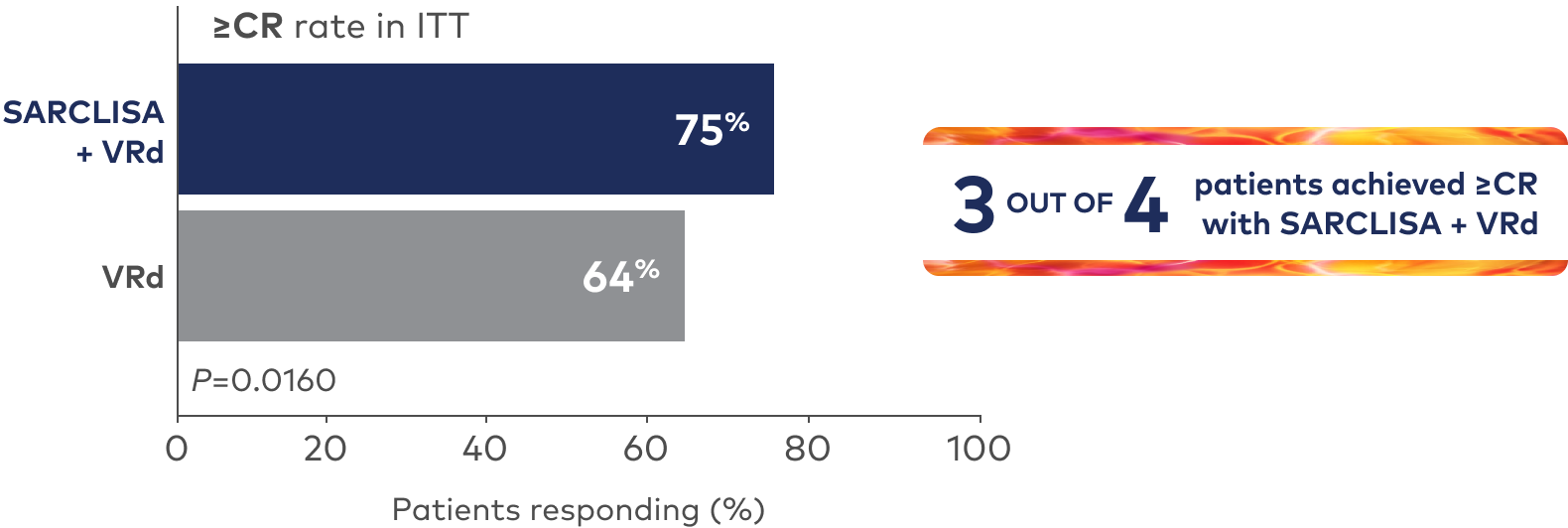
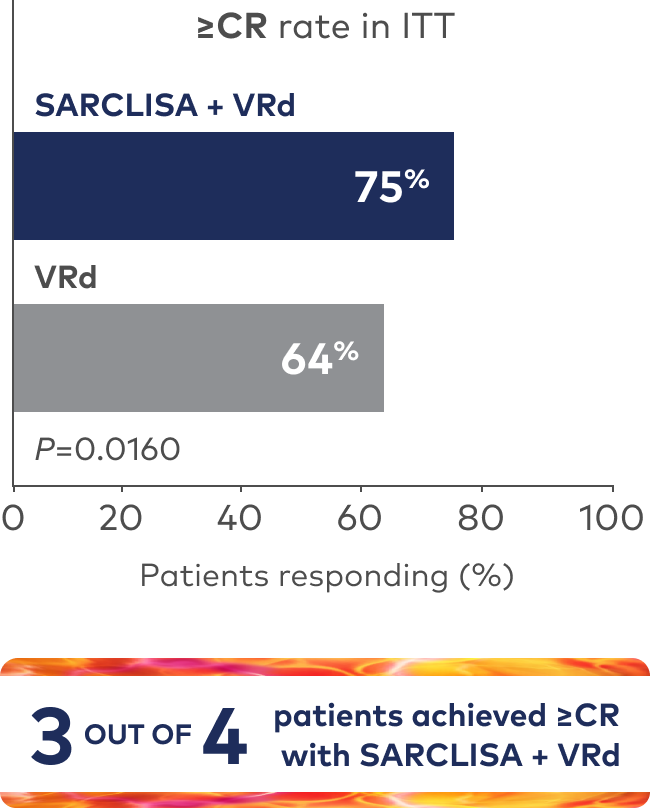
Deeper rate of MRD- with SARCLISA + VRd vs VRd alone1

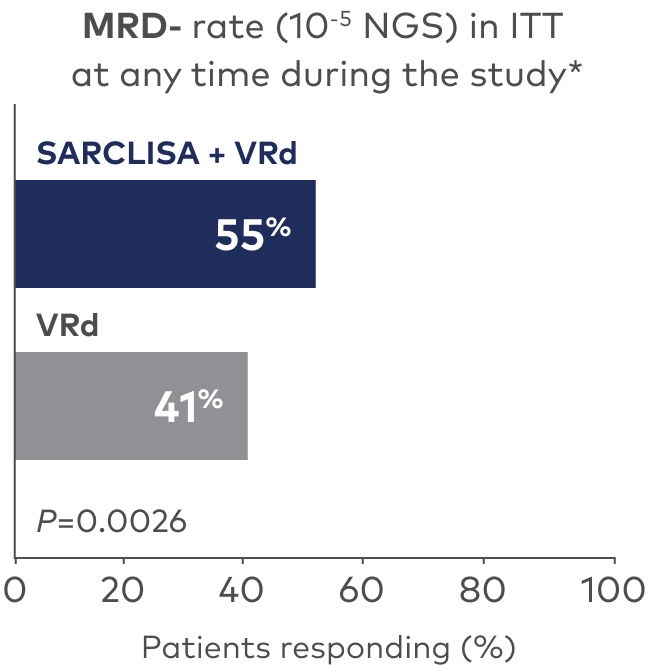
MRD- and sustained MRD- in ≥VGPR (ITT)1,2,7
- 58% of patients receiving SARCLISA + VRd (n=265) achieved MRD- in ≥VGPR (ITT) vs 44% with VRd alone (n=181)
- at a median follow-up of 60 months (46.8% with SARCLISA + VRd vs 24.3% with VRd [ITT])
Limitations: Data for MRD- and sustained MRD- in ≥VGPR (ITT) ≥1 year were not multiplicity controlled and results are descriptive. Definitive conclusions cannot be made.
*Patients achieved both MRD- and a response of ≥CR.1
CR=complete response; ITT=intent to treat; MRD-=minimal (or measurable) residual disease negative/negativity; NGS=next-generation sequencing; VGPR=very good partial response.
Estimated overall survival from IMROZ (ITT)†
At 60-month median follow-up1,2:
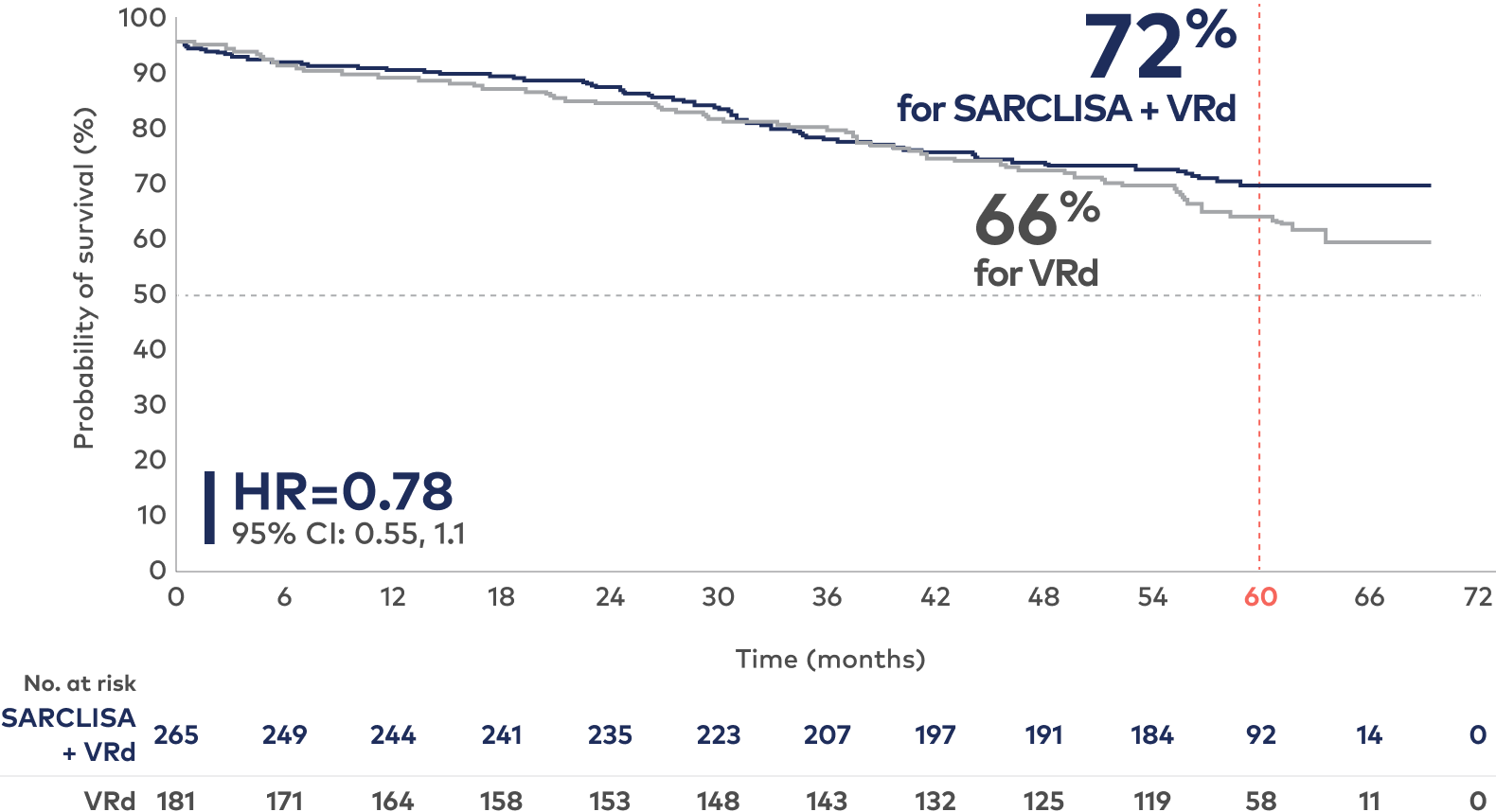
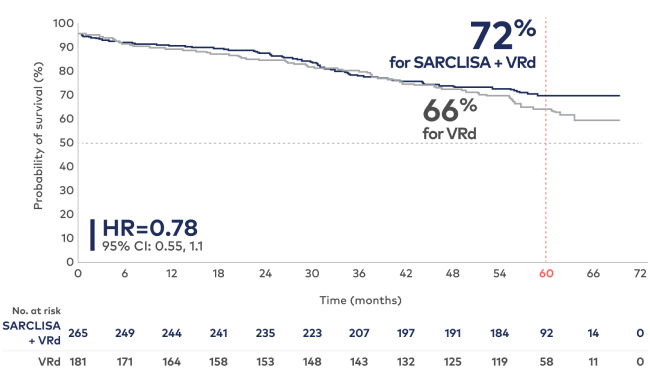
Study limitations: The OS results are not yet mature. OS analysis was not adjusted for multiplicity and conclusions cannot be made.
Median OS was not reached for either treatment group. At a median follow-up time of 60 months, 26% of patients in the SARCLISA + VRd group and 32.6% of patients in the VRd group had died; HR=0.78 (95% CI: 0.55, 1.1).1
In the continuous phase, patients randomized to the VRd arm who experienced disease progression during the Rd treatment period could cross over to receive SARCLISA + Rd.2
†Kaplan-Meier estimate.
OS=overall survival; Rd=lenalidomide and dexamethasone.
SARCLISA: The first and only FDA-approved anti-CD38 + VRd therapy in patients with NDMM not eligible for transplant1,8


IMROZ is a large, randomized, open-label, multicenter, phase 3 trial that compared SARCLISA + VRd to VRd alone1,2
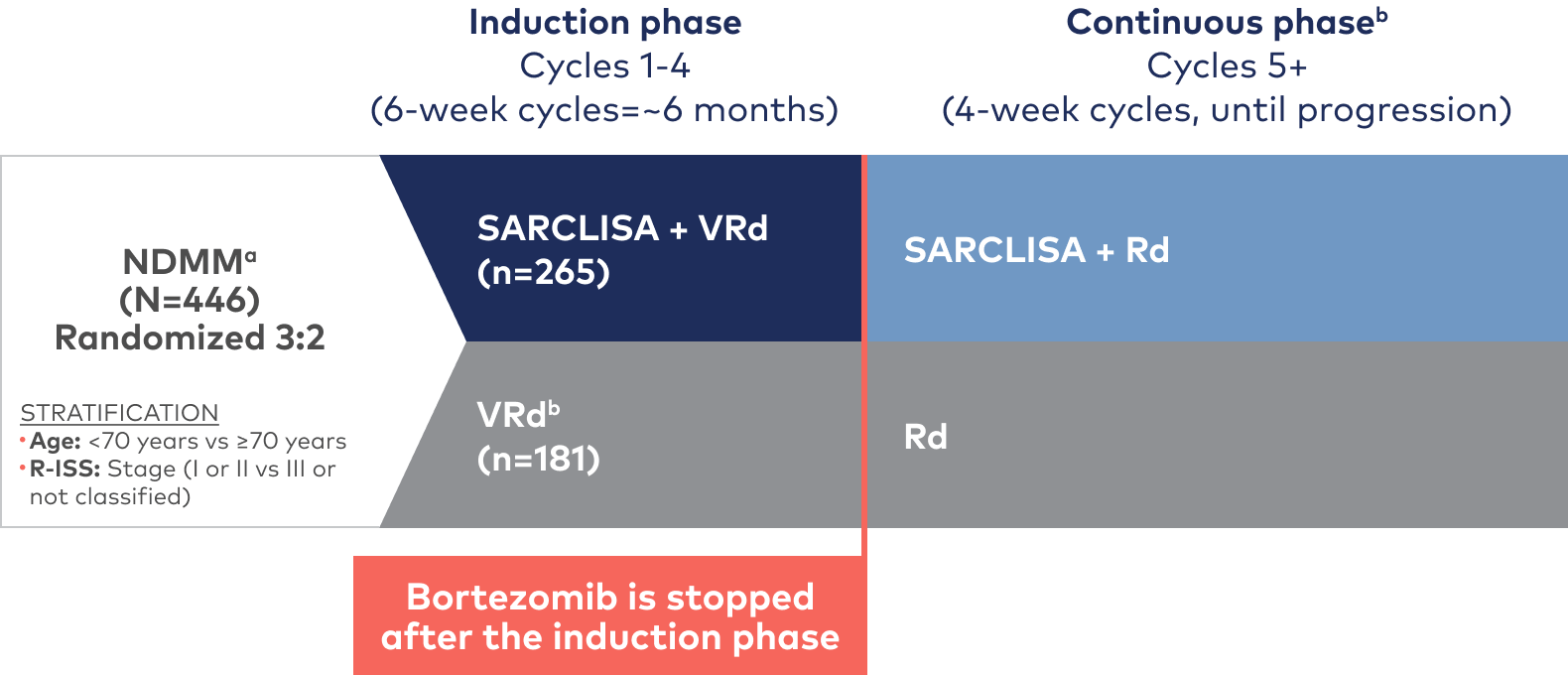
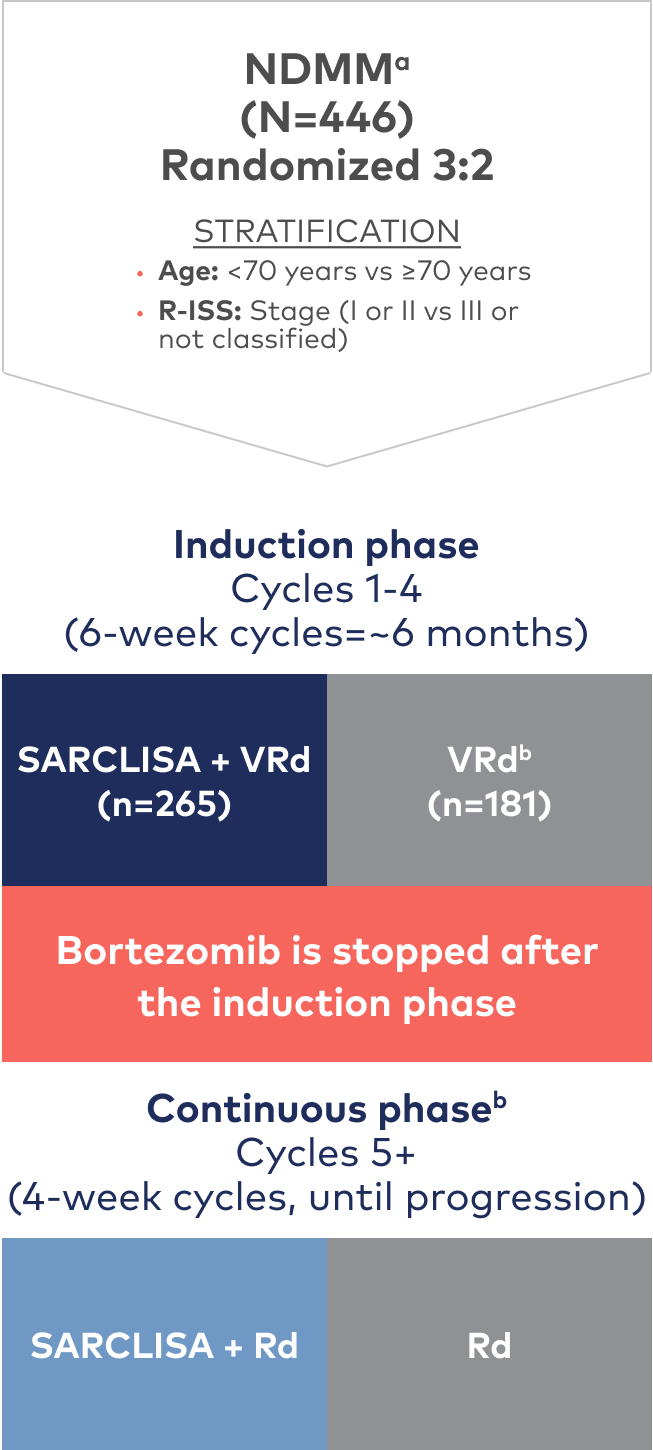
IMROZ was conducted in 2 phases: an induction phase and a continuous phase. Patients received either SARCLISA 10 mg/kg administered as an IV infusion in combination with VRd or VRd alone in four 42-day cycles. Following the induction phase, bortezomib was discontinued for both treatment arms. SARCLISA was given weekly in the first cycle and every 2 weeks during cycles 2 to 17, and monthly for cycle 18+.1
During the induction phase, bortezomib was administered subcutaneously at the dose of 1.3 mg/m2 on days 1, 4, 8, 11, 22, 25, 29, and 32 of each cycle. Lenalidomide was administered orally at the dose of 25 mg/day from day 1 to 14 and from day 22 to 35 of each cycle. Dexamethasone (IV on the days of SARCLISA infusions, and orally on the other days) 20 mg/day was given on days 1, 2, 4, 5, 8, 9, 11, 12, 15, 22, 23, 25, 26, 29, 30, 32, and 33 of each cycle. In the continuous phase, lenalidomide was administered orally at the dose of 25 mg/day from day 1 to 21 of each cycle. Dexamethasone (IV on the days of SARCLISA infusions, and orally on the other days) 20 mg/day was given on days 1, 8, 15, and 22 of each cycle.1
PRIMARY ENDPOINT: PFS1‡
Key secondary endpoints: ≥CR,
MRD- in ≥CR, ≥VGPR, and OS2

‡PFS results were assessed by an IRC based on central laboratory data for M-protein and central radiologic imaging review using the IMWG criteria.1
aThe interim analysis of PFS was performed after 162 events of disease progression or death had occurred (73% of the planned 222 events for the final analysis).2
bIn the continuous phase, patients randomized to the VRd arm who experienced progressive disease during the Rd treatment period could cross over to receive SARCLISA + Rd.2
IV=intravenous.
Key patient characteristics in the IMROZ trial1,2
In the IMROZ trial (N=446):
- The median age was 72 years
- 4% of patients were <65 years, 68% were between 65 and 74 years, and 28% were ≥75 years
- ECOG PS was 0 or 1 for 89% of patients, and >1 for 11% of patients
- At study start, patient breakdown by R-ISS was as follows: I in 23%, II in 64%, and III in 10% of patients
- Overall, 17% of patients had high-risk chromosomal abnormalities at study entry; del(17p), t(4;14), and t(14;16) were present in 5%, 9%, and 2% of patients, respectively. In addition, 1q21+ was present in 37% of patients
- Patient characteristics were similar between the 2 treatment arms
ECOG PS=Eastern Cooperative Oncology Group performance status; R-ISS=Revised International Staging System.
IMROZ Trial Summary Brochure
An informative brochure that includes the IMROZ trial results for SARCLISA + VRd vs VRd alone