SCHEDULE
Dosing Information for SARCLISA
Recommended dose1
10 mg/kgactual body weight administered as an IV infusion in combination with VRd, or Kd, or Pd
250-mLfixed infusion volume
Treatment is repeated until disease progression or unacceptable toxicity
IV=intravenous; Kd=carfilzomib and dexamethasone; Pd=pomalidomide and dexamethasone; VRd=bortezomib, lenalidomide, dexamethasone.
Infusion time decreases to 75 minutes by the third infusion1
Incremental escalation of the infusion rate should be considered only in the absence of IRRs.
first infusion
second infusion
SUBSEQUENT INFUSIONS
IRR=infusion-related reaction.
NDMM dosing schedule
RRMM dosing schedule
For patients with NDMM not eligible for transplant
SARCLISA dosing schedule in combination with VRd1
The recommended dose of SARCLISA is 10 mg/kg administered as an IV infusion at a fixed infusion volume of 250 mL in combination with VRd.
Dosing frequency for SARCLISA transitions to once monthly*
INDUCTION PHASE
(6-WEEK CYCLES)†
CYCLE 1
WEEKLY
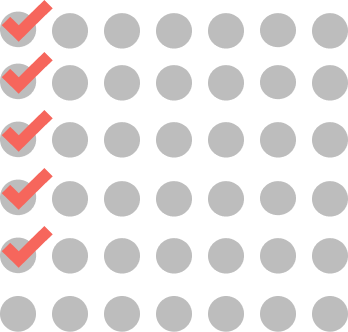
5 doses
CYCLES 2-4
ONCE EVERY 2 WEEKS
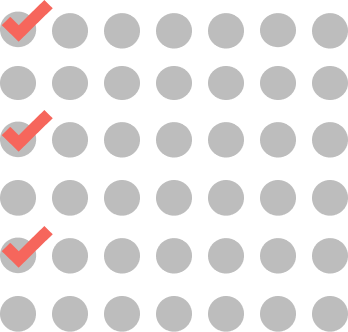
3 doses every cycle
CONTINUOUS PHASE
(4-WEEK CYCLES)‡
CYCLES 5-17
ONCE EVERY 2 WEEKS
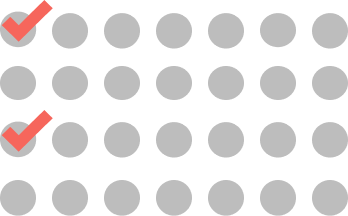
2 doses every cycle
CYCLE 18
AND BEYOND
ONCE EVERY 4 WEEKS
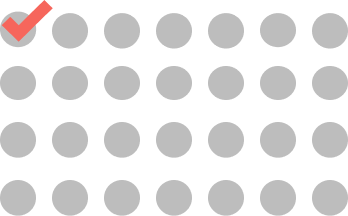
1 dose every cycle
For dosing instructions for combination agents administered with SARCLISA, refer to the trial page for IMROZ and the respective manufacturer's Prescribing Information.
View the RRMM dosing schedule*Initiates after cycle 17.
†Induction phase cycles=42 days each.
‡Continuous phase cycles=28 days each.
SARCLISA dosing schedule in combination with Kd or Pd1
The recommended dose of SARCLISA is 10 mg/kg administered as an IV infusion at a fixed infusion volume of 250 mL in combination with either Kd or Pd.
Dosing frequency for SARCLISA decreases after cycle 1
WEEKLY DOSING TRANSITIONS TO EVERY OTHER WEEK AFTER THE FIRST CYCLE
CYCLE 1
WEEKLY
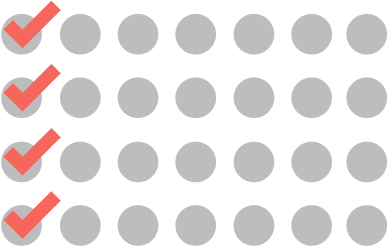
4 doses
Cycle 2 and beyond
ONCE EVERY 2 WEEKS
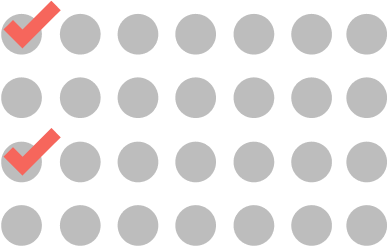
2 doses every cycle
On days where both SARCLISA and carfilzomib are administered, administer dexamethasone first, followed by SARCLISA infusion, then followed by carfilzomib infusion.
For additional dosing instructions for Kd and Pd, refer to the trial pages for IKEMA and ICARIA-MM, and the respective manufacturer's Prescribing Information.
View the NDMM dosing schedulePremedication and antimicrobial prophylaxis1


Administer the following premedications prior to SARCLISA infusion to reduce the risk and severity of IRRs:
Dexamethasone
SARCLISA + Kd: 20 mg (IV on the days of SARCLISA and/or carfilzomib infusions, and orally on the other days)
SARCLISA + Pd: 40 mg orally or IV (or 20 mg orally or IV for patients ≥75 years of age)
SARCLISA + VRd: 20 mg (IV on the days of SARCLISA infusions, and orally on the other days)
Acetaminophen
650 mg to 1000 mg orally (or equivalent)
H2 antagonist
Institution-preferred agent
Diphenhydramine
25 mg to 50 mg orally or IV (or equivalent). The IV route is preferred for at least the first 4 infusions
The above recommended dose of dexamethasone (orally or IV) corresponds to the total dose to be administered only once before infusion as part of the premedication and of the backbone treatment, before SARCLISA and carfilzomib, SARCLISA and pomalidomide, or SARCLISA, bortezomib, and lenalidomide administration.
Administer the recommended premedication agents 15 to 60 minutes prior to starting a SARCLISA infusion. No post-infusion medications are required for SARCLISA.
Recommended antimicrobial prophylaxis
Initiate antibacterial and antiviral prophylaxis (such as herpes zoster prophylaxis) if needed based on standard guidelines.
Infusion rates of SARCLISA administration1
Calculate the dose (mg) of required SARCLISA based on actual patient weight (measured prior to each cycle to have the administered dose adjusted accordingly). Note that more than one vial of SARCLISA may be necessary to obtain the required dose for the patient.
Incremental escalation of the infusion rate should be considered only in the absence of IRRs.
SARCLISA should be administered by a healthcare professional with immediate access to emergency equipment and appropriate medical support to manage IRRs if they occur.
Administering SARCLISA1
Prepare the solution for infusion using an aseptic technique
- Administer the infusion solution by IV
infusion using an IV tubing infusion set (in PE, PVC with or without DEHP, PBD, or PU)
with a 0.22-micron in-line filter (PES, polysulfone, or nylon)
- The infusion solution should be administered for a period of time that will depend on the infusion rate (see the table above)
- Use prepared SARCLISA infusion solution within 48 hours when stored refrigerated at 36°F to 46°F (2°C to 8°C), followed by 8 hours (including the infusion time) at room temperature
- Do not administer SARCLISA infusion solution concomitantly in the same IV line with other agents
- On days where both SARCLISA and carfilzomib are administered, administer dexamethasone first, followed by SARCLISA infusion, then followed by carfilzomib infusion


