FINAL ANALYSIS:
44 months median follow-up
SARCLISA + CARFILZOMIB
AND DEXAMETHASONE (Kd)
IKEMA: SARCLISA + Kd vs
Kd Alone in RRMM
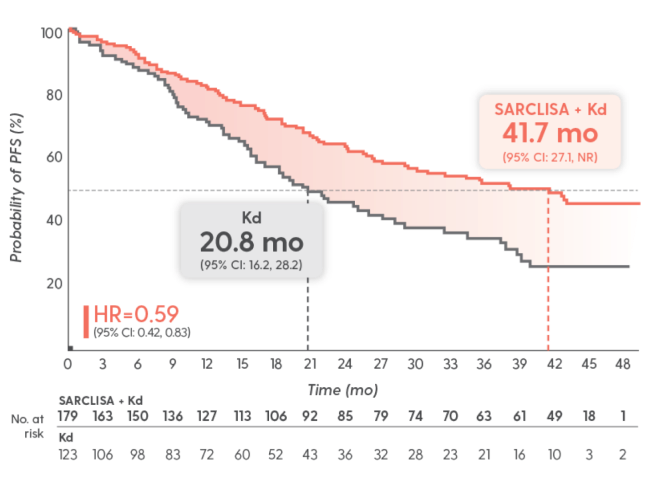
Unprecedented results: ~42 months mPFS with SARCLISA + Kd
Longest ever reported in a phase 3 trial that included lenalidomide-refractory patients1-7*
*Based on a review of published phase 3 trials that included lenalidomide-refractory patients with RRMM. Interpret with caution, as various factors, including patient population, differ between trials.
PFS results were assessed by an IRC, based on central laboratory data for M-protein, and central radiologic imaging review using IMWG criteria. A preplanned interim analysis was conducted when 65% of 159 PFS events were observed. P value is not reported as this is a non-inferential analysis of the primary endpoint that was met at the time of the interim analysis.1,8
Superior PFS vs Kd at
interim analysis
(median follow-up of 20.7 months)1
SARCLISA + Kd: mPFS NR
Kd: mPFS 20.27 months1
HR=0.548
(95% CI: 0.37, 0.82; P=0.0032)1
Final analysis: A prespecified final analysis was conducted when 159 PFS events were observed, with a median follow-up of 44 months.2
IMWG=International Myeloma Working Group; IRC=independent response committee; mPFS=median progression-free survival; M-protein=monoclonal protein; NR=not reached; PFS=progression-free survival; RRMM=relapsed or refractory multiple myeloma.
NCCN
CATEGORY 1
PREFERRED
National Comprehensive Cancer Network® (NCCN®) recommends isatuximab-irfc (SARCLISA) in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma as a Category 1 Preferred option in combination with carfilzomib and dexamethasone or with pomalidomide and dexamethasone10:
- For early relapses (1-3 prior therapies)†
- Option for patients refractory to either lenalidomide or bortezomib
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
Recommendation for isatuximab-irfc (SARCLISA) in combination with carfilzomib and dexamethasone based on results of interim analysis.
†After 2 prior therapies including lenalidomide and a proteasome inhibitor for isatuximab-irfc in combination with pomalidomide and dexamethasone.
NCCN=National Comprehensive Cancer Network® (NCCN®).
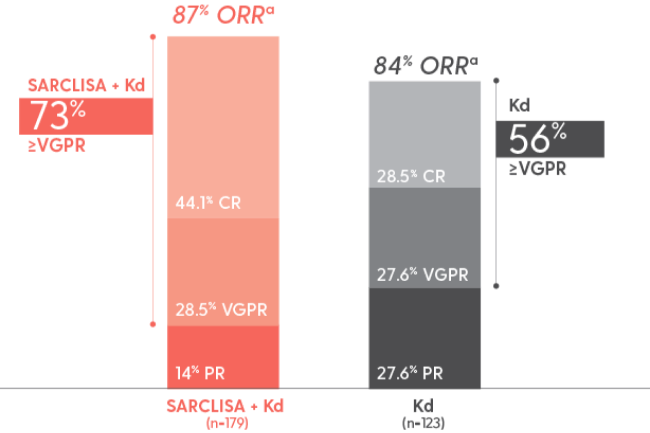
Patients in the IKEMA trial achieved deep responses2‡
High rates of ≥VGPR were seen in IKEMA
As ORR did not reach statistical significance, ≥VGPR and CR were not tested for significance
Interim analysis (20.7 months median follow-up)1,8
- ORR:
-
- — 86.6% (95% CI: 0.81, 0.91) with SARCLISA + Kd
- — 82.9% (95% CI: 0.75, 0.89) with Kd alone
- — P=0.3859; 95% CI estimated using the Clopper-Pearson method
- ≥VGPR: 72.6% with SARCLISA + Kd vs 56.1% with Kd alone
- CR: 39.7% with SARCLISA + Kd vs 27.6% with Kd alone
- MRD-: 30% with SARCLISA + Kd (95% CI: 0.23, 0.37) vs 13% with Kd alone (95% CI: 0.08, 0.20)
The median time to first response among responders was 1.08 months in the SARCLISA + Kd arm and 1.12 months in the Kd arm.8
‡A response of ≥VGPR.
asCR, CR, VGPR, and PR were evaluated by the IRC using the IMWG response criteria. Results are based on a prespecified final analysis with a median follow-up time of 44 months.1
CR=complete response; MRD-=minimal (or measurable) residual disease negative/negativity; ORR=overall response rate; PR=partial response; sCR=stringent complete response; VGPR=very good partial response.
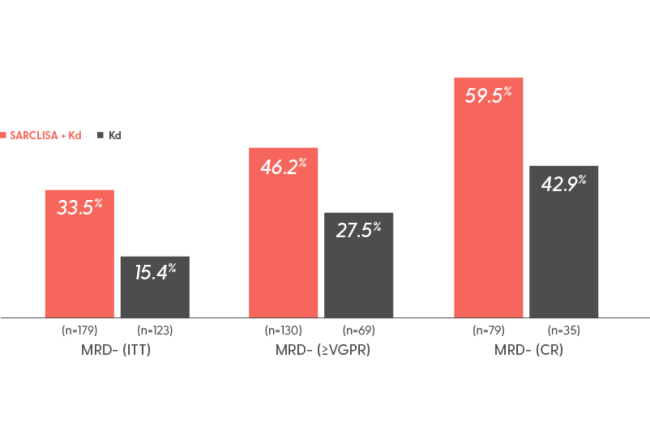
6 out of 10 patients in CR achieved MRD- with SARCLISA + Kd2,9
Rate of MRD- by adaptive NGS at 10-5§
Interim analysis (20.7 months median follow-up): MRD- rate in the ITT population was 30% with SARCLISA + Kd (95% CI: 0.23, 0.37) vs 13% with Kd alone (95% CI: 0.08, 0.20)1,8
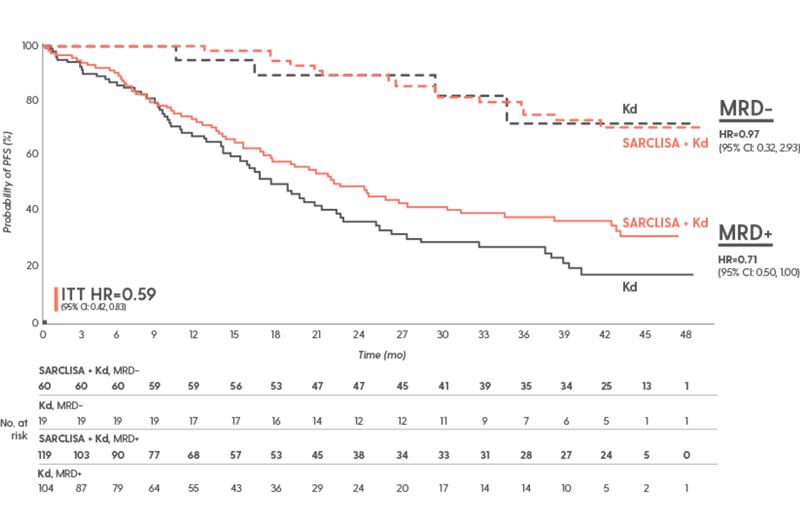
In IKEMA, achieving MRD- translated into prolonged PFS8
PFS by MRD status and treatment arm at the final analysis
PFS results were assessed by an IRC, based on central laboratory data for M-protein, and central radiologic imaging review using the IMWG criteria.1
Study limitations
According to the FDA, using MRD to assess clinical benefit of a multiple myeloma treatment should only be assessed in patients who achieve a CR or sCR. In the IKEMA trial, MRD was assessed in patients who achieved ≥VGPR. Additionally, there was an amount of missing data that did not meet the FDA’s threshold for label inclusion. This analysis requires cautious interpretation and clinical significance of these data is unknown.
§Based on a sensitivity level of 10-5 by NGS in patients who achieved ≥VGPR.8
ITT=intent to treat; MRD+=minimal (or measurable) residual disease positive/positivity; NGS=next-generation sequencing.
Subgroup data for SARCLISA + Kd8
Consistent PFS results were seen across almost all subgroups with SARCLISA + Kd
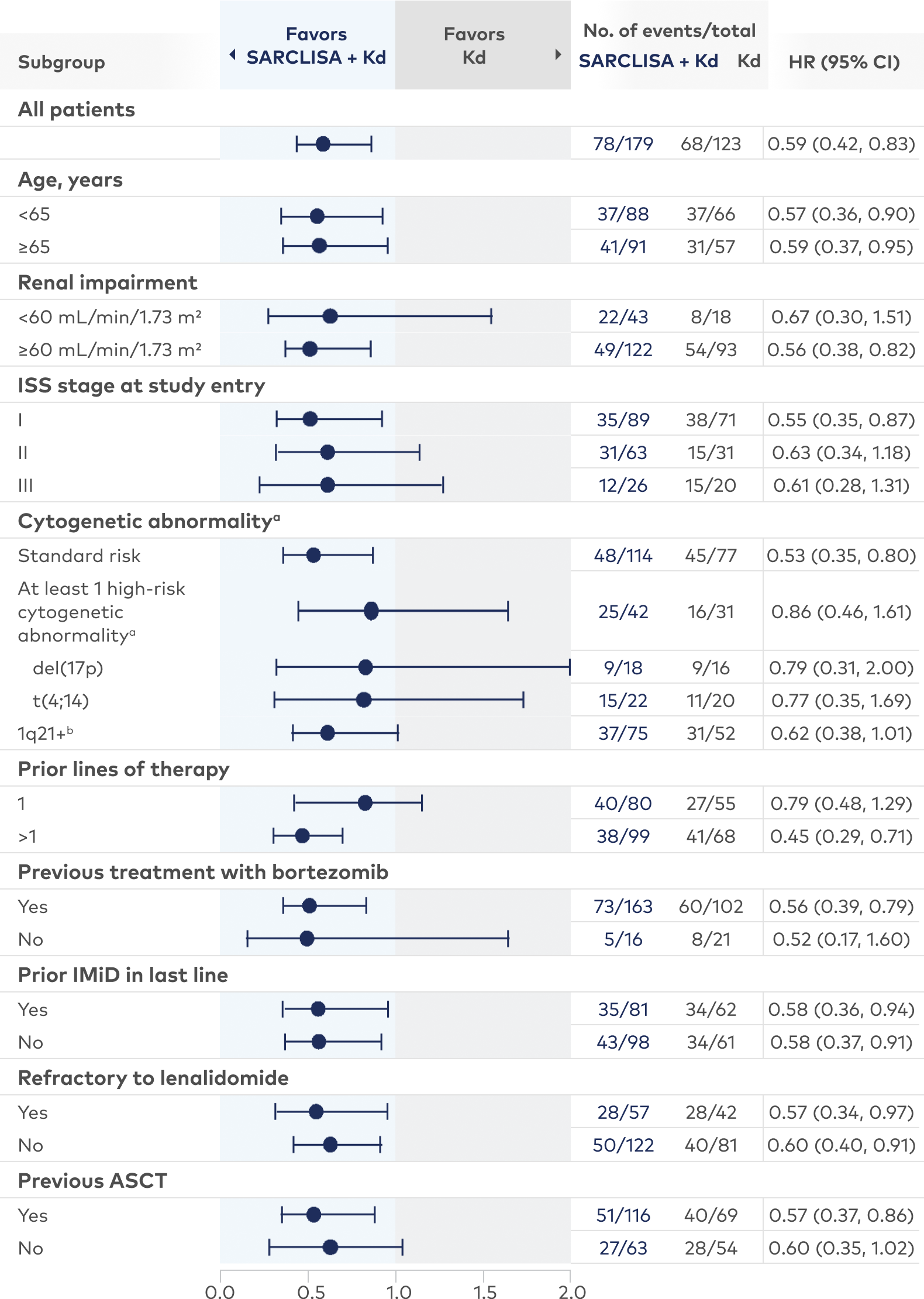
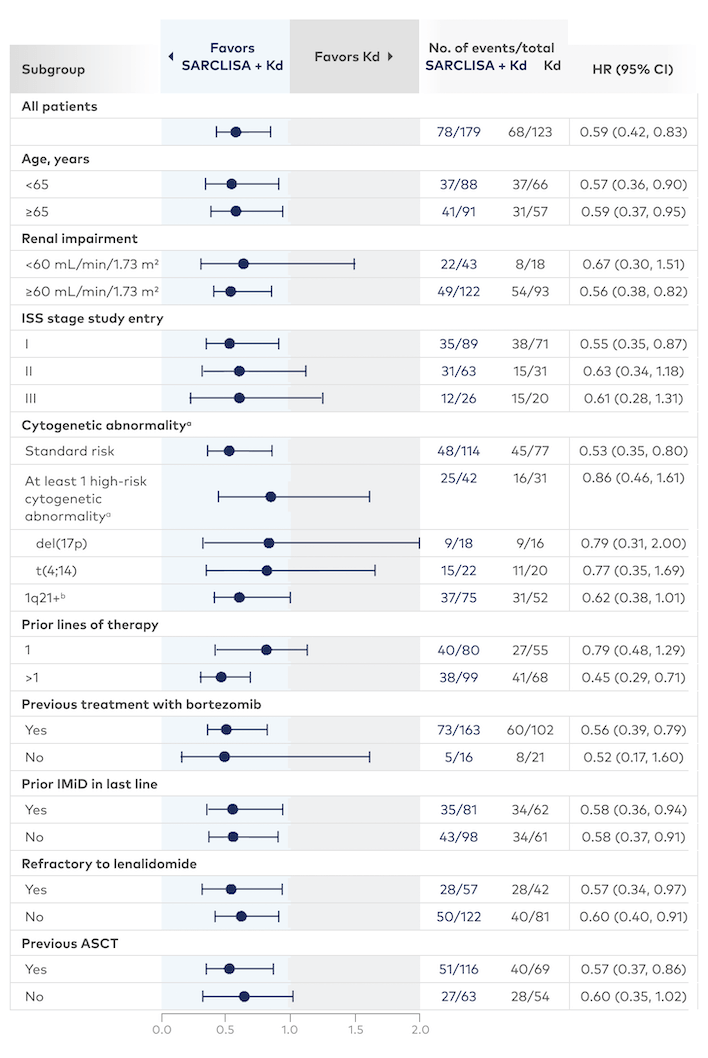
aHigh-risk cytogenetic status was defined as the presence of del(17p) and/or t(4;14) and/or t(14;16). Chromosomal abnormality was considered positive if present in ≥30% of analyzed plasma cells, except for del(17p), where the threshold was ≥50%; please note that due to the low number of patients with t(14;16), the HR could not be calculated.
b1q21+ was also analyzed and was considered positive if there were ≥3 copies in ≥30% of analyzed plasma cells.
Study limitations
All subgroups were prespecified except the lenalidomide-refractory subgroup. Subgroups were not powered to show differences between treatment arms.
ASCT=autologous stem cell transplant; IMiD=immunomodulatory drug; ISS=International Staging System.
Reduction in the risk of disease progression in patients treated with SARCLISA + Kd vs Kd alone
Older age ≥65 years of age
41%
HR=0.59 (95% CI: 0.37, 0.95)
Refractory to lenalidomide
43%
HR=0.57 (95% CI: 0.34, 0.97)
Renal impairment eGFR <60 mL/
min/1.73 m2
33%
HR=0.67 (95% CI: 0.30, 1.51)
Presence of 1q21+¶
38%
HR=0.62 (95% CI: 0.38, 1.01)
Study limitations
All subgroups were prespecified except the lenalidomide-refractory subgroup. Subgroups were not powered to show differences between treatment arms.
¶1q21+ was considered positive if there were ≥3 copies in ≥30% of analyzed plasma cells.
eGFR=estimated glomerular filtration rate.
IKEMA trial: SARCLISA + Kd vs Kd alone


Evaluated in 302 patients in a phase 3, multicenter, multinational, randomized, open-label study1,8
sarclisaa + Kdb
(n=179)
Kdb
(n=123)
Treatment was administered in 28-day cycles until disease progression or unacceptable toxicity.
PRIMARY ENDPOINT: PFSǁ
Key secondary endpoints: ORR, ≥VGPR, CR, MRD negativity, OS

aSARCLISA 10 mg/kg was administered as an IV infusion weekly in the first cycle and every 2 weeks thereafter.
bCarfilzomib was administered as an IV infusion during cycle 1 at a dose of 20 mg/m2 on days 1 and 2, and at 56 mg/m2 on days 8, 9, 15, and 16; during subsequent cycles, it was administered at 56 mg/m2 on days 1, 2, 8, 9, 15, and 16. Dexamethasone (IV on the days of SARCLISA and/or carfilzomib infusions, and orally on the other days) 20 mg was given on days 1, 2, 8, 9, 15, 16, 22, and 23 of each 28-day cycle.
IIPFS results were assessed by an IRC, based on central laboratory data for M-protein, and central radiologic imaging review using the IMWG criteria. An interim analysis was conducted when 65% of the 159 PFS events (ie, 103 events) were observed. A prespecified final analysis was conducted when 159 PFS events were observed, with a median follow-up of 44 months.
IV=intravenous; OS=overall survival.
The IKEMA trial included a broad and diverse patient population1,8,11
Patient factors
Treatment history
43%
Baseline characteristics were balanced across both treatment arms
(n=179)
(n=123)
aImpaired renal function is defined as eGFR <60 mL/min/1.73 m2.1
bHigh-risk cytogenetic status is defined as the presence of del(17p) and/or t(4;14) and/or t(14;16). Chromosomal abnormality was considered positive if present in ≥30% of analyzed plasma cells, except for del(17p), where the threshold is ≥50%.8
c1q21+ was also analyzed and was considered positive if there were ≥3 copies in ≥30% of analyzed plasma cells.8
dInclusion criteria for the IKEMA study specified 1 to 3 prior lines of therapy; however, 3 patients (1.7%) and 2 patients (1.6%) had >3 prior lines in the SARCLISA + Kd and Kd arms, respectively.8
ECOG PS=Eastern Cooperative Oncology Group performance status; PI=proteasome inhibitor.